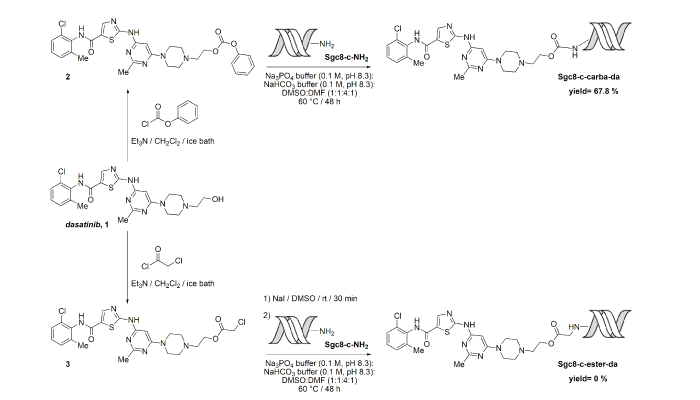
Chemical conjugations of Sgc8-c with the lymphoma drug dasatinib to generate selective biotherapeutics
RESEARCH ARTICLE
![]()
ISSN: 2514-3247
Aptamers (2021), Vol 5, 15-21
Published online: 18 October 2021
Full Text (Sicco ~840kb) | (Sicco Supplementary Data ~1108kb) (PubMed Central Record HTML) | (PubMed) | (References)
Estefanía Sicco 1, Lucía Almeida 1, María Moreno 2, Victoria Calzada 1,*, Hugo Cerecetto 1,*
1 Área de Radiofarmacia, Centro de Investigaciones Nucleares, Facultad de Ciencias, Universidad de la República, Mataojo 2055, 11400 Montevideo, Uruguay
2 Departamento de Desarrollo Biotecnológico, Instituto de Higiene, Facultad de Medicina, Universidad de la República, 11600 Montevideo, Uruguay
*Correspondence to: Victoria Calzada, Email: vcalzada@cin.edu.uy; Hugo Cerecetto, Email: hcerecetto@cin.edu.uy
Received: 16 July 2021 | Revised: 13 October 2021 | Accepted: 18 October 2021
© Copyright The Author(s). This is an open access article, published under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0). This license permits non-commercial use, distribution and reproduction of this article, provided the original work is appropriately acknowledged, with correct citation details.
ABSTRACT
The conjugation of drugs to target therapeutics has become a promising method that could improve the efficacy of therapy and reduce side effects. Herein, we describe the efforts to covalently link the anti-lymphoma agent dasatinib to the truncated aptamer Sgc8-c, expecting the new hybrids to specifically damage lymphoma cells but with minimal toxicity towards non-target cells. Two linkages, ester and carbamate, with variable pH labilities were used to connect Sgc8-c with dasatinib. Different reaction conditions were studied by varying the solvent, time, temperature, heat source, pH and counter-ions. Each product from the reaction mixture was analysed by qualitative electrospray ionization time-of-flight mass spectrometry, identifying the nucleic acid modifications formed under the different experimental conditions. Among the reactions, depurinations from the 3´-extreme mainly occurred as lateral processes. Preparation of the carbamate-linked Sgc8-c–dasatinib hybrid Sgc8-c-carb-da was successful but the ester-linked hybrid only produced lateral undesired products. The potential biotherapeutic Sgc8-c-carb-da displayed the ability to trigger dasatinib at endosomal pH, which is optimal because this could be the aptamer’s cellular uptake route.
KEYWORDS: biotherapeutics, Sgc8-c, dasatinib, drug delivery, synthesis, depurination
