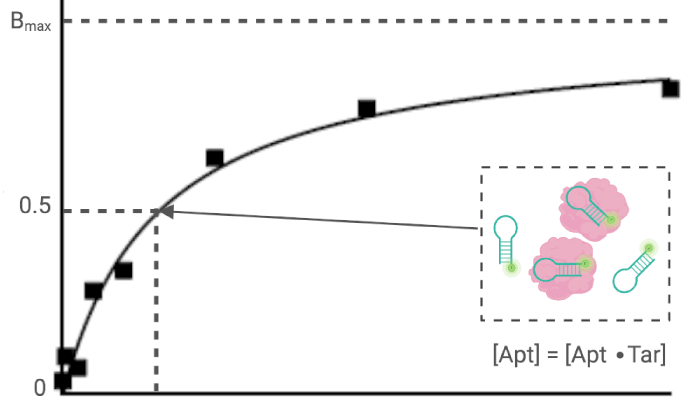
Quantification of aptamer-protein binding with fluorescence anisotropy
METHOD/PROTOCOL
![]()
ISSN: 2514-3247
Aptamers (2021), Vol 5, 1-6
Published online: 18 January 2021
Full Text (Hirka ~721kb) | (PubMed Central Record HTML) | (PubMed) | (References)
Serhii Hirka1, Maureen McKeague1,2,*
1Department of Chemistry, McGill University, 801 Sherbrooke Street West, Montréal, Québec, H3A 0B8, Canada
2Department of Pharmacology and Therapeutics, McGill University, 3655 Prom. Sir-William-Osler, Montréal, Québec, H3G 1Y6, Canada
*Correspondence to: *Correspondence to: Maureen McKeague, Email: maureen.mckeague@mcgill.ca
Received: 28 September 2020 | Revised: 15 January 2021 | Accepted: 18 January 2021
© Copyright The Author(s). This is an open access article, published under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0). This license permits non-commercial use, distribution and reproduction of this article, provided the original work is appropriately acknowledged, with correct citation details.
ABSTRACT
At the turn of the 21st century, aptamers selected in vitro have secured their place in the mainstream study of nucleic acids and are commonly evolved via a process termed SELEX. While new sequences can be isolated from SELEX in as little as a 14-day time period, characterizing putative aptamer sequences poses a greater challenge and often goes unreported in the literature. Rigorous characterization and validation of individual sequences are required for reliable reporting of aptamer sequences with high affinity and specificity to their targets. Here, we present a protocol for the use of fluorescence anisotropy to characterize aptamers towards protein targets. This protocol also serves as an introduction for new aptamer researchers, in particular students, who wish to understand and perform aptamer binding experiments.
KEYWORDS: Aptamer, fluorescence anisotropy, dissociation constant, protein targets
