Development of transferrin receptor aptamers as drug delivery vehicles for the treatment of brain metastases
REVIEW ARTICLE
![]()
ISSN: 2514-3247
Aptamers (2018), Vol 2, 15-27
Published online: 27 April 2018
Full Text (PDF ~3667kb) | (PubMed Central Record HTML) | (PubMed) | (References)
Joanna Macdonald1, Giovanni Mandarano1, Rakesh Naduvile Veedu2,3, and Sarah Shigdar1,4,*
1School of Medicine Deakin University, Geelong, VIC, Australia- 3216
2Centre for Comparative Genomics, Murdoch University, Perth, WA, Australia-6150
3Perron Institute for Neurological and Translational Science, Perth, WA, Australia – 6009
4Centre for Molecular and Medical Research, Deakin University, Geelong, VIC, Australia- 3216
*Correspondence to: Sarah Shigdar, Email: sarah.shigdar@deakin.edu.au
Received: 15 December 2017 | Revised: 24 April 2018 | Accepted: 27 April 2018
© Copyright The Author(s). This is an open access article, published under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0). This license permits non-commercial use, distribution and reproduction of this article, provided the original work is appropriately acknowledged, with correct citation details.
ABSTRACT
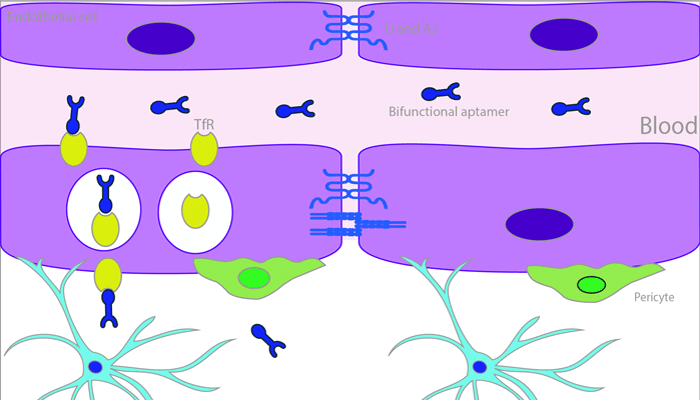
Affecting approximately up to 10-40% of all cancer patients, the prognosis for patients suffering from metastatic brain tumours is poor. Treatment of these metastatic tumours is greatly hindered by the presence of the blood brain barrier which restricts the overwhelming majority of small molecules from entering the brain. A novel approach to overcome this barrier is to target receptor mediated transport mechanisms present on the endothelial cell membranes, in particular the transferrin receptor. Given their specificity, safety profile and stability, nucleic acid-based therapeutics are ideal for this purpose. This review explores the development of bifunctional aptamers for the treatment of brain metastases.
KEYWORDS: Blood-brain-barrier, transferrin receptor, brain metastases, aptamers, bifunctional aptamer, drug delivery