Optical control of aptamer-based sensors using a photocleavable linker
METHOD
![]()
ISSN: 2514-3247
Aptamers (2019), Vol 3, 10-16
Published online: 20 December 2019
Full Text (TanHeemstra ~606kb) | (PubMed Central Record HTML) | (PubMed) | (References)
Zhesen Tan, Trevor A Feagin, Jennifer M Heemstra*
Department of Chemistry, Emory University, Atlanta, Georgia, USA
*Correspondence to: Jennifer Heemstra, Email: jen.heemstra@emory.edu, Tel: + 404 727 7766
Received: 15 June 2019 | Revised: 05 December 2019 | Accepted: 06 December 2019
© Copyright The Author(s). This is an open access article, published under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0). This license permits non-commercial use, distribution and reproduction of this article, provided the original work is appropriately acknowledged, with correct citation details.
ABSTRACT
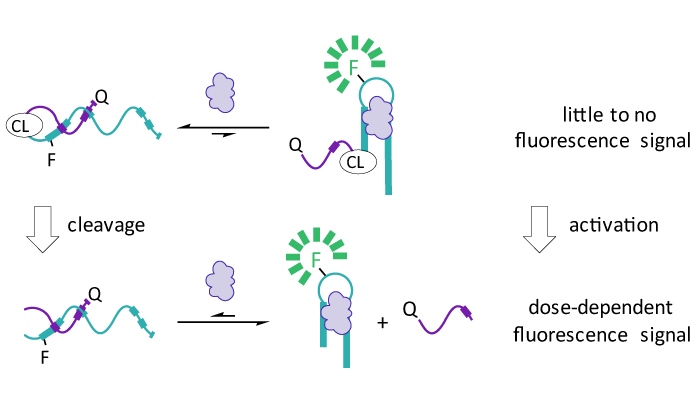
Aptamer-based sensors rely on the ability of nucleic acids to bind to target molecules with high affinity and specificity via molecular recognition. Due to their ease of production and the high thermal stability of nucleic acids, aptamer-based sensors are emerging as attractive candidates for detecting a wide range of chemical and biological targets. A current limitation of aptamer-based sensors, however, is the inability to control their activity in a time-resolved manner, limiting their utility in applications that require precise temporal control over function. Here, we demonstrate that temporal control over structure-switching (SS) aptamer sensors can be achieved using an equilibrium-shifting photocleavable linker that is placed between the aptamer and complementary strand. Installation of this linker significantly increases the effective molarity of the complementary strand, temporarily shifting the equilibrium of the sensor to disfavor target binding, and thus rendering the sensor functionally inert to the target molecule. To restore activity, the linker can be cleaved by UV irradiation, which returns the sensor into its functional equilibrium state, and allows for a dose-dependent response in the presence of target. To demonstrate the generalizability of our photocleavable linker design, we have shown that the linker can be grafted onto a different SS biosensor and still provide time-resolved control over activity. This represents a key benefit of our approach, as it allows the caging strategy to be easily adapted for use with aptamer sensors for a wide range of target molecules. Together, this research demonstrates that precise temporal control over aptamer sensors can be achieved without modifying the actual nucleotide sequence of the sensor, creating a generalizable method for control of sensor function. We anticipate that this will be particularly useful when deploying aptamer sensors in complex biological environments.
Keywords: Photocleavable, aptamer sensors, covalently-caged biosensors, method