Experimental and mathematical evidence that thrombin-binding aptamers form a 1 aptamer:2 protein complex
RESEARCH ARTICLE
![]()
ISSN: 2514-3247
Aptamers (2018), Vol 2, 64-73
Published online: 10 October 2018
Full Text (PDF ~1270kb) (Mears-Suppl-Information ~567kb) | (PubMed Central Record HTML) | (PubMed) | (References)
Kepler S Mears1, Daniel L Markus1, Oluwadamilare Ogunjimi1 and Rebecca J Whelan1,2,*
1Department of Chemistry and Biochemistry, Oberlin College, USA
2Department of Chemistry and Biochemistry, University of Notre Dame, USA
*Correspondence to: Rebecca Whelan, Email: rwhelan1@nd.edu, Tel: +574-631-1853, Fax: 574-631-6652
Received: 07 September 2018 | Revised: 04 October 2018 | Accepted: 07 October 2018
© Copyright The Author(s). This is an open access article, published under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0). This license permits non-commercial use, distribution and reproduction of this article, provided the original work is appropriately acknowledged, with correct citation details.
ABSTRACT
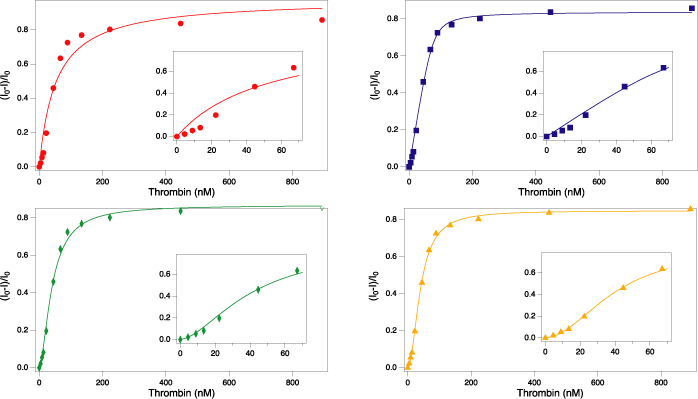
The thrombin-binding 15mer and 29mer ssDNA aptamers are a widely used model system. Despite their ubiquity, controversies persist regarding the nature of the aptamer-protein interactions. Reported affinities vary widely; the role of metal ions in binding is unclear; the structure of the complex is contested. We interrogated the effects of instrument, buffer, and mathematical model on apparent affinities of thrombin aptamers for their target. Instrumental method had a pronounced effect on affinity constants for the 15mer and marginal effect the apparent affinity of the 29mer. Buffer composition and ionic environment did not have significant effects. Affinity probe capillary electrophoresis experiments revealed distinct peaks from samples of 29mer aptamer and thrombin, supporting the model of a 1 aptamer:2 protein complex. Fits to high quality data with five mathematical models further support this stoichiometry, as the binding of both aptamers was best described by the Hill equation with Hill coefficients > 1. Our results indicate that the instrumental method and mathematical model influence apparent affinity of thrombin aptamers and that both aptamers bind thrombin in a 1 aptamer: 2 protein stoichiometry through an induced fit mechanism.
KEYWORDS: Thrombin, DNA aptamers, thrombin-binding aptamers, affinity assays, mathematical model, Hill equation